Toxicology Screening Systems Market Expected to Surpass USD 13.6 Billion by 2036 as Regulatory Mandates
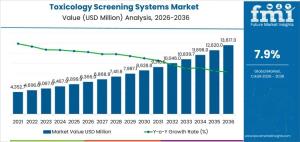
The toxicology screening systems market is projected to grow from USD 6,366.0 million in 2026 to USD 13,617.0 million by 2036, at a CAGR of 7.9%.
NEWARK, DE, UNITED STATES, January 16, 2026 /EINPresswire.com/ -- The global toxicology screening systems market is entering a high-growth phase, with a projected valuation of USD 6,366.0 million in 2026. New industry analysis indicates that the sector will expand to USD 13,617.0 million by 2036, representing a robust compound annual growth rate (CAGR) of 7.9%.
This momentum is fundamentally anchored in the shifting landscape of public safety and clinical diagnostics. As of early 2026, the demand for high-specificity testing is being driven by the integration of expanded drug panels—including fentanyl and novel psychoactive substances—into standard workplace and clinical protocols. Hospitals, forensic labs, and occupational health services are increasingly pivoting toward automated platforms that provide legally defensible results under intensifying audit pressure.
Redefining Analytical Standards through Advanced Confirmation
Modern toxicology workflows are moving away from simple "yes/no" qualitative screens toward comprehensive quantitative analysis. The market is currently seeing a significant shift in capital allocation toward systems that bridge the gap between initial triage and definitive legal proof.
Request For Sample Report | Customize Report |purchase Full Report - https://www.futuremarketinsights.com/reports/sample/rep-gb-31384
Core Drivers of Institutional Utilization:
• Expansion of Testing Panels: Regulatory updates in 2026, including the addition of fentanyl to Department of Transportation (DOT) urine and oral fluid panels, are mandating broader screening capabilities.
• Precision and Legal Defensibility: High-complexity labs are prioritizing LC-MS/MS systems to virtually eliminate cross-reactivity and false positives, ensuring results withstand forensic and court scrutiny.
• Clinical Decision Support: In emergency and psychiatric care, the need for rapid, broad-spectrum identification of toxicants is critical for guided acute treatment and risk mitigation.
• Automation and Throughput: Robotic sample preparation and automated LIS integration are becoming essential for centralized reference labs managing high-volume compliance programs.
Market Segmentation: Confirmatory Systems and Workplace Compliance
The technology landscape is increasingly defined by the hierarchy of "screen-then-confirm" workflows. LC-MS/MS confirmatory systems currently lead the device category with a 24.0% share, reflecting their role as the "gold standard" for forensic and clinical verification.
• Workplace Drug Testing (28.0% Application Share): Routine monitoring and federally mandated compliance programs remain the largest source of sustained testing volume.
• Clinical and Acute Care (24.0% Share): Rapid-response screening in emergency departments is a vital growth segment, particularly in response to the global polysubstance use crisis.
• Reference Laboratories (26.0% Channel Share): Centralized facilities continue to dominate due to their ability to validate complex methods and offer economies of scale to smaller clinics.
Regional Dynamics: India and Brazil Lead Growth Acceleration
While the United States and Germany remain the most mature markets with steady replacement cycles, emerging economies are seeing the highest growth rates as they modernize their forensic and healthcare infrastructure.
• India (10.4% CAGR): Growth is powered by the massive expansion of private diagnostic chains and a push to modernize forensic laboratory throughput to handle rising caseloads.
• Brazil (9.9% CAGR): Strategic government funding for state-level laboratory upgrades and stricter workplace drug testing in regulated industries are driving adoption.
• China (9.4% CAGR): High-volume hospital automation and national drug safety monitoring programs are fueling the deployment of domestically manufactured, cost-competitive platforms.
• United States (6.5% CAGR): Demand is sustained by new 2026 testing mandates and a shift toward oral fluid testing as a more flexible alternative to traditional urine collection.
Strategic Competitive Landscape
The competitive environment is led by industry giants including Abbott, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific, and Beckman Coulter. Differentiation in 2026 is centered on "menu breadth"—the ability of a single platform to detect an ever-evolving list of designer drugs and metabolites with minimal recalibration.
As the industry moves toward 2030, the focus is expected to shift toward further miniaturization and the integration of AI-driven analytics to interpret complex chromatograms. This evolution will allow laboratories to maintain high quality standards and operational efficiency despite global skilled-staffing constraints.
About the Toxicology Screening Systems Market Report
This press release is based on market data last updated on January 15, 2026. The report provides a comprehensive evaluation of the global analytical toxicology landscape, covering technology transitions, regional regulatory shifts, and infrastructure investment trends across 25+ countries.
Similar Industry Reports
Toxicology Drug Screening Market
https://www.futuremarketinsights.com/reports/toxicology-drug-screening-market
Oral Screening Systems Market
https://www.futuremarketinsights.com/reports/oral-screening-systems-market
Systems Administration Management Tools Market
https://www.futuremarketinsights.com/reports/systems-administration-management-tools-market
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.